2021 ACR/VF Guideline for the Management of Giant Cell Arteritis and Takayasu Arteritis
These recommendations provide guidance regarding the evaluation and management of patients with GCA and TAK, including diagnostic strategies, use of pharmacologic agents, and surgical interventions.
Download Complete Guideline
https://www.youtube.com/watch?v=69A1UGWqsdYhttps://www.youtube.com/watch?v=mnqp1rdeqb8
General Information
Mehrdad Maz,1![]() Sharon A. Chung,2 Andy Abril,3 Carol A. Langford,4 Mark Gorelik,5 Gordon Guyatt,6 Amy M. Archer,7 Doyt L. Conn,8
Sharon A. Chung,2 Andy Abril,3 Carol A. Langford,4 Mark Gorelik,5 Gordon Guyatt,6 Amy M. Archer,7 Doyt L. Conn,8![]() Kathy A. Full,9 Peter C. Grayson,10
Kathy A. Full,9 Peter C. Grayson,10![]() Maria F. Ibarra,11 Lisa F. Imundo,5 Susan Kim,2 Peter A. Merkel,12
Maria F. Ibarra,11 Lisa F. Imundo,5 Susan Kim,2 Peter A. Merkel,12![]() Rennie L. Rhee,12
Rennie L. Rhee,12![]() Philip Seo,13 John H. Stone,14
Philip Seo,13 John H. Stone,14![]() Sangeeta Sule,15
Sangeeta Sule,15![]() Robert P. Sundel,16 Omar I. Vitobaldi,17 Ann Warner,18 Kevin Byram,19 Anisha B. Dua,7 Nedaa Husainat,20
Robert P. Sundel,16 Omar I. Vitobaldi,17 Ann Warner,18 Kevin Byram,19 Anisha B. Dua,7 Nedaa Husainat,20![]() Karen E. James,21 Mohamad A. Kalot,22
Karen E. James,21 Mohamad A. Kalot,22![]() Yih Chang Lin,23 Jason M. Springer,1
Yih Chang Lin,23 Jason M. Springer,1![]() Marat Turgunbaev,24 Alexandra Villa-Forte,4 Amy S. Turner,24
Marat Turgunbaev,24 Alexandra Villa-Forte,4 Amy S. Turner,24 and Reem A. Mustafa25
and Reem A. Mustafa25![]()
Guidelines and recommendations developed and/or endorsed by the American College of Rheumatology (ACR) are intended to provide guidance for particular patterns of practice and not to dictate the care of a particular patient. The ACR considers adherence to the recommendations within this guideline to be voluntary, with the ultimate determination regarding their application to be made by the physician in light of each patient’s individual circumstances. Guidelines and recommendations are intended to promote beneficial or desirable outcomes but cannot guarantee any specific outcome. Guidelines and recommendations developed and endorsed by the ACR are subject to periodic revision as warranted by the evolution of medical knowledge, technology, and practice. ACR recommendations are not intended to dictate payment or insurance decisions, and drug formularies or other third-party analyses that cite ACR guidelines should state this. These recommendations cannot adequately convey all uncertainties and nuances of patient care.
The American College of Rheumatology is an independent, professional, medical and scientific society that does not guarantee, warrant, or endorse any commercial product or service.
Objective. To provide evidence-based recommendations and expert guidance for the management of giant cell arteritis (GCA) and Takayasu arteritis (TAK) as exemplars of large vessel vasculitis.
Methods. Clinical questions regarding diagnostic testing, treatment, and management were developed in the population, intervention, comparator, and outcome (PICO) format for GCA and TAK (27 for GCA, 27 for TAK). Systematic literature reviews were conducted for each PICO question. The Grading of Recommendations Assessment, Development and Evaluation methodology was used to rate the quality of the evidence. Recommendations were developed by the Voting Panel, comprising adult and pediatric rheumatologists and patients. Each recommendation required ≥70% consensus among the Voting Panel.
Results. We present 22 recommendations and 2 ungraded position statements for GCA, and 20 recommendations and 1 ungraded position statement for TAK. These recommendations and statements address clinical questions relating to the use of diagnostic testing, including imaging, treatments, and surgical interventions in GCA and TAK. Recommendations for GCA include support for the use of glucocorticoid-sparing immunosuppressive agents and the use of imaging to identify large vessel involvement. Recommendations for TAK include the use of nonglucocorticoid immunosuppressive agents with glucocorticoids as initial therapy. There were only 2 strong recommendations; the remaining recommendations were conditional due to the low quality of evidence available for most PICO questions.
Conclusion. These recommendations provide guidance regarding the evaluation and management of patients with GCA and TAK, including diagnostic strategies, use of pharmacologic agents, and surgical interventions.
Giant cell arteritis (GCA) and Takayasu arteritis (TAK) are systemic vasculitides that primarily affect large- and medium-sized vessels (1). GCA can present with both cranial and extracranial manifestations. Cranial manifestations include headaches, scalp tenderness, vision loss, and jaw claudication. Large vessel (“extracranial”) involvement results in arterial stenosis and aneurysms, causing absent pulses and limb claudication (2). GCA is more common in individuals of Northern European descent who are older than 50 years of age. Diagnosis is based on clinical presentation, pathologic abnormalities on temporal artery biopsy, and/or evidence of large vessel involvement on vascular imaging (1–6). Glucocorticoids are the mainstay treatment for GCA, but tocilizumab has been approved by the US Food and Drug Administration for the treatment of GCA (7,8).
TAK causes granulomatous inflammation of the aorta and its branches. It is more common in younger women (9,10). Clinical manifestations include constitutional symptoms, elevated levels of inflammation markers, and arterial stenosis and/or aneurysms resulting in limb claudication and absent pulses (11). Treatment options include glucocorticoids, nonglucocorticoid immunosuppressive agents, and surgical management of vascular abnormalities (12).
As GCA and TAK share clinical manifestations, similar questions arise regarding their treatment and management. Recent studies have broadened treatment options for GCA, and vascular imaging is increasingly used for diagnosis and management. This guideline was developed to provide evidence-based recommendations for the evaluation and management of GCA and TAK.
This guideline followed the American College of Rheumatology (ACR) guideline development process (https://www. rheumatology.org/Practice-Quality/Clinical-Support/Clinical- Practice-Guidelines) using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology to rate the quality of evidence and develop recommendations (13–15). ACR policy guided the management of conflicts of interest and disclosures (https://www.rheumatology.org/ Practice-Quality/Clinical-Support/Clinical-Practice-Guidelines/ Vasculitis). Supplementary Appendix 1 (available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/ doi/10.1002/art.41774/abstract) presents a detailed descrip- tion of the methods. Briefly, the Literature Review team undertook systematic literature reviews for predetermined questions specifying the clinical population, intervention, comparator, and outcomes (PICO). An in-person Patient Panel of 11 individuals with different types of vasculitis (3 patients with GCA or TAK) was moderated by a member of the Literature Review team (ABD). This Patient Panel reviewed the evidence report (along with a summary and interpretation by the moderator) and provided patient perspectives and preferences about their personal experiences regarding clinical and treatment aspects of their disease. The Voting Panel comprised 9 adult rheumatologists, 5 pediatric rheumatologists, and 2 patients; they reviewed the Literature Review team’s evidence summaries and, bearing in mind the Patient Panel’s deliberations, formulated and voted on recommendations. A recommendation required ≥70% consensus among the Voting Panel.
How to interpret the recommendations
A strong recommendation is typically supported by moderate- to high-quality evidence (e.g., multiple randomized controlled trials). For a strong recommendation, the recommended course of action would apply to all or almost all patients. Only a small proportion of clinicians/patients would not want to follow the recommendation. In rare instances, a strong recommendation may be based on very low– to low-certainty evidence. For example, an intervention may be strongly recommended if it is considered low-cost, without harms, and the consequence of not performing the intervention may be catastrophic. An intervention may be strongly recommended against if there is high certainty that the intervention will lead to more harm than the comparison with very low or low certainty about its benefit (16).
A conditional recommendation is generally supported by lower-quality evidence or a close balance between desirable and undesirable outcomes. For a conditional recommendation, the recommended course of action would apply to the majority of the patients, but the alternative is a reasonable consideration. Conditional recommendations always warrant a shared decision-making approach. We specify conditions under which the alternative may be considered.
In some instances, the committee found that the evidence for a particular PICO question did not support a graded recommendation or did not favor one intervention over another. However, the Voting Panel believed that the PICO question addressed a commonly encountered clinical question which has not been fully clarified and requires further investigation, and thus felt that providing guidance for this question was warranted. For these situations, we present “ungraded position statements,” which reflect general views of the Voting Panel.
In this evidence-based guideline, we explicitly used the best evidence available and present that in a transparent manner for the clinician reader/user (10). In some instances, this includes randomized trials in which the interventions under consideration are directly compared. The GRADE system rates evidence that comes exclusively from the collective experience of the Voting Panel and Patient Panel members as “very low– quality” evidence (15).
For each recommendation, details regarding the PICO questions and the GRADE evidence tables can be found in Supplementary Appendix 2 (http://onlinelibrary.wiley.com/doi/10.1002/ art.41774/abstract).
This guideline presents the ACR/Vasculitis Foundation recommendations for the use of diagnostic testing, treatment, clinical and laboratory monitoring, and surgical intervention for patients with GCA or TAK. Overarching themes of the recommendations include the preference, in the US, for temporal artery biopsy over cranial imaging studies for the diagnosis of GCA, the use of large vessel imaging for GCA and TAK for diagnosis and disease monitoring, and limiting glucocorticoid exposure in order to minimize toxicity. Almost all recommendations are conditional due to low- quality evidence, reflecting the paucity of randomized clinical trials in these diseases.
Our recommendations regarding the use of temporal artery imaging differ from those presented by the European Alliance of Associations for Rheumatology (EULAR). In its recommendations regarding the use of imaging in large vessel vasculitis, EULAR indicates that the diagnosis of GCA may be made with a positive imaging test (e.g., temporal artery ultrasound or MRI of the cranial vessels), without additional testing such as temporal artery biopsy (103). However, the imaging recommendations presented by EULAR assume adequate expertise with these modalities. In the US, there is limited experience with temporal artery ultrasound and MRI of the cranial vessels as a diagnostic replacement for temporal artery biopsy, and thus, we continue to recommend temporal artery biopsy as the diagnostic test of choice at this time. However, we hope and anticipate that as experience with imaging of the temporal arteries to detect GCA (e.g., temporal artery ultra- sound, MRI, and/or FDG-PET) increases in the US, patients will be able to benefit from these diagnostic tests. Also, in contrast to EULAR, we favor initial treatment of GCA with glucocorticoids and a glucocorticoid-sparing agent, given the well-recognized toxicity of glucocorticoids (104,105).
When reviewing the data abstracted for the PICO questions, it was clear that many critical clinical questions remain unanswered for GCA and TAK, and the lack of sufficient clinical evidence for these questions is reflected in the ungraded position statements presented in this guideline. For example, the optimal duration of therapy for any treatment and how best to monitor disease status is unknown. Few glucocorticoid-sparing agents have been identified through high-quality data. Accurate and validated indicators of disease activity have not been established or widely used for GCA or TAK. Interpretation of imaging studies in GCA and TAK can be challenging, and the clinical significance of persistent vascular wall inflammation during clinically quiescent disease is unclear.
Given these critical gaps in knowledge, we encourage additional research into the management of GCA and TAK. Studies that may greatly benefit patient care include the following:
1) translational studies contributing to the understanding of disease pathogenesis to facilitate development of more targeted therapies;
2) randomized clinical trials identifying new therapeutic options for the management of GCA and TAK;
3) randomized clinical trials comparing the effectiveness of currently used immunosuppressive therapies; and
4) longitudinal studies with biospecimen collection and routine vascular imaging to identify biomarkers of disease activity, indicators of disease prognosis, and the clinical sequelae of abnormalities identified on vascular imaging.
We are hopeful that additional investigations into GCA and TAK will enable a more tailored approach to disease management in order to improve outcomes and minimize treatment toxicities.
We thank Anne M. Ferris, MBBS, Ora Gewurz-Singer, MD, Rula Hajj-Ali, MD, Eric Matteson, MD, MPH, Robert F. Spiera, MD, Linda Wagner-Weiner, MD, MS, and Kenneth J. Warrington, MD, for serving on the Expert Panel. We thank Antoine G. Sreih, MD and Gary S. Hoffman, MD, MS, for their contributions during the early phases of this project as members of the Core Team. Dr. Hoffman’s participation ended July 2018 due to personal reasons. Dr. Sreih’s involvement ended in December 2018 when he became primarily employed by industry, which precluded his continued participation in this project. We thank Joyce Kullman (Vasculitis Foundation) for her assistance with recruitment for the Patient Panel. We thank the patients who (along with authors Kathy A. Full and Omar I. Vitobaldi) participated in the Patient Panel meeting: Jane Ascroft, Scott A. Brunton, Dedra DeMarco, Thomas Fitzpatrick, Jenn Gordon, Maria S. Mckay, Sandra Nye, Stephanie Sakson, and Ben Wilson. We thank Robin Arnold, Catherine E. Najem, MD, MSCE, and Amit Aakash Shah, MD, MPH, for their assistance with the literature review. We thank the ACR staff, including Ms Regina Parker, for assistance in organizing the face-to- face meeting and coordinating the administrative aspects of the project, and Ms Robin Lane for assistance in manuscript preparation. We thank Ms Janet Waters for help in developing the literature search strategy and performing the initial literature search, and Ms Janet Joyce for performing the update searches.
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Drs. Maz, Chung, and Abril had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Maz, Chung, Abril, Langford, Gorelik, Guyatt, Archer, Full, Grayson, Merkel, Seo, Stone, Sule, Sundel, Vitobaldi, Turner, Mustafa.
Acquisition of data. Maz, Chung, Langford, Abril, Gorelik, Full, Imundo, Kim, Merkel, Stone, Vitobaldi, Byram, Dua, Husainat, James, Kalot, Lin, Springer, Turgunbaev, Villa-Forte, Turner, Mustafa.
Analysis and interpretation of data. Maz, Chung, Langford, Abril, Gorelik, Archer, Conn, Full, Grayson, Ibarra, Imundo, Kim, Merkel, Rhee, Seo, Stone, Vitobaldi, Warner, Byram, Dua, Husainat, Kalot, Lin, Springer, Turgunbaev, Mustafa.
- Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et 2012 revised International Chapel Hill Consensus Conference nomenclature of vasculitides. Arthritis Rheum 2013;65:1–11.
- Weyand CM, Goronzy Giant-cell arteritis and polymyalgia rheumatica [letter]. N Engl J Med 2014;371:1653.
- Gonzalez-Gay MA, Vazquez-Rodriguez TR, Lopez-Diaz MJ, Miranda-Filloy JA, Gonzalez-Juanatey C, Martin J, et Epidemiology of giant cell arteritis and polymyalgia rheumatica [review]. Arthritis Rheum 2009;61:1454–61.
- Koster MJ, Matteson EL, Warrington Large-vessel giant cell arteritis: diagnosis, monitoring and management [review]. Rheumatology (Oxford) 2018;57 Suppl 2:ii32–42.
- Mukhtyar C, Guillevin L, Cid MC, Dasgupta B, de Groot K, Gross W, et EULAR recommendations for the management of large vessel vasculitis. Ann Rheum Dis 2009;68:318–23.
- Salvarani C, Crowson CS, O’Fallon WM, Hunder GG, Gabriel Reappraisal of the epidemiology of giant cell arteritis in Olmsted County, Minnesota, over a fifty-year period. Arthritis Rheum 2004;51:264–8.
- Aiello PD, Trautmann JC, McPhee TJ, Kunselman AR, Hunder Visual prognosis in giant cell arteritis. Ophthalmology 1993;100:550–5.
- Stone JH, Tuckwell K, Dimonaco S, Klearman M, Aringer M, Blockmans D, et al. Trial of tocilizumab in giant-cell arteritis. N Engl J Med 2017;377:317–28.
- Hall S, Barr W, Lie JT, Stanson AW, Kazmier FJ, Hunder Takayasu arteritis: a study of 32 North American patients. Medicine (Baltimore) 1985;64:89–99.
- Schmidt J, Kermani TA, Bacani AK, Crowson CS, Cooper LT, Matteson EL, et Diagnostic features, treatment, and outcomes of Takayasu arteritis in a US cohort of 126 patients. Mayo Clin Proc 2013;88:822–30.
- Sanchez-Alvarez C, Mertz LE, Thomas CS, Cochuyt JJ, Abril Demographic, clinical, and radiologic characteristics of a cohort of patients with Takayasu arteritis. Am J Med 2019;132:647–51.
- Labarca C, Makol A, Crowson CS, Kermani TA, Matteson EL, Warrington Retrospective comparison of open versus endovascular procedures for Takayasu arteritis. J Rheumatol 2016;43:427–32.
- Alexander PE, Li SA, Gionfriddo MR, Stoltzfus RJ, Neumann I, Brito JP, et Senior GRADE methodologists encounter challenges as part of WHO guideline development panels: an inductive content analysis. J Clin Epidemiol 2016;70:123–8.
- Andrews J, Guyatt G, Oxman AD, Alderson P, Dahm P, Falck-Ytter Y, et GRADE guidelines: 14. Going from evidence to recommendations: the significance and presentation of recommendations. J Clin Epidemiol 2013;66:719–25.
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso- Coello P, et GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–6.
- Alexander PE, Gionfriddo MR, Li SA, Bero L, Stoltzfus RJ, Neumann I, et A number of factors explain why WHO guideline developers make strong recommendations inconsistent with GRADE guidance. J Clin Epidemiol 2016;70:111–22.
- Durling B, Toren A, Patel V, Gilberg S, Weis E, Jordan Incidence of discordant temporal artery biopsy in the diagnosis of giant cell arteritis. Can J Ophthalmol 2014;49:157–61.
- Mahr A, Saba M, Kambouchner M, Polivka M, Baudrimont M, Brochériou I, et Temporal artery biopsy for diagnosing giant cell arteritis: the longer, the better? Ann Rheum Dis 2006;65:826–8.
- Roth AM, Milsow L, Keltner The ultimate diagnoses of patients undergoing temporal artery biopsies. Arch Ophthalmol 1984;102:901–3.
- Achkar AA, Lie JT, Hunder GG, O’Fallon WM, Gabriel How does previous corticosteroid treatment affect the biopsy findings in giant cell (temporal) arteritis? Ann Intern Med 1994;120:987–92.
- Allison MC, Gallagher Temporal artery biopsy and corticosteroid treatment. Ann Rheum Dis 1984;43:416–7.
- Breuer GS, Nesher R, Nesher Negative temporal artery biopsies: eventual diagnoses and features of patients with biopsy-negative giant cell arteritis compared to patients without arteritis. Clin Exp Rheumatol 2008;26:1103–6.
- Bury D, Joseph J, Dawson Does preoperative steroid treatment affect the histology in giant cell (cranial) arteritis? J Clin Pathol 2012;65:1138–40.
- Gonzalez-Gay MA, Garcia-Porrua C, Llorca J, Gonzalez-Louzao C, Rodriguez-Ledo Biopsy-negative giant cell arteritis: clinical spectrum and predictive factors for positive temporal artery biopsy. Semin Arthritis Rheum 2001;30:249–56.
- Hall S, Persellin S, Lie JT, O’Brien PC, Kurland LT, Hunder The therapeutic impact of temporal artery biopsy. Lancet 1983;2:1217–20.
- Le K, Bools LM, Lynn AB, Clancy TV, Hooks WB III, Hope The effect of temporal artery biopsy on the treatment of temporal arteritis. Am J Surg 2015;209:338–41.
- Ray-Chaudhuri N, Kine DA, Tijani SO, Parums DV, Cartilidge N, Strong NP, et Effect of prior steroid treatment on temporal artery biopsy findings in giant cell arteritis. Br J Ophthalmol 2002;86:530–2.
- Maleszewski JJ, Younge BR, Fritzlen JT, Hunder GG, Goronzy JJ, Warrington KJ, et Clinical and pathological evolution of giant cell arteritis: a prospective study of follow-up temporal artery biopsies in 40 treated patients. Mod Pathol 2017;30:788–96.
- Bley TA, Uhl M, Carew J, Markl M, Schmidt D, Peter HH, et Diagnostic value of high-resolution MR imaging in giant cell arteritis. AJNR Am J Neuroradiol 2007;28:1722–7.
- Bowling K, Rait J, Atkinson J, Srinivas Temporal artery biopsy in the diagnosis of giant cell arteritis: does the end justify the means? Ann Med Surg (Lond) 2017;20:1–5.
- Hussain O, McKay A, Fairburn K, Doyle P, Orr Diagnosis of giant cell arteritis: when should we biopsy the temporal artery? Br J Oral Maxillofac Surg 2016;54:327–30.
- Luqmani R, Lee E, Singh S, Gillett M, Schmidt WA, Bradburn M, et The role of ultrasound compared to biopsy of temporal arteries in the diagnosis and treatment of giant cell arteritis (TABUL): a diagnostic accuracy and cost-effectiveness study. Health Technol Assess 2016;20:1–238.
- Yuksel V, Guclu O, Tastekin E, Halici U, Huseyin S, Inal V, et Clinical correlation of biopsy results in patients with temporal arteritis. Rev Assoc Med Bras (1992) 2017;63:953–6.
- Ghinoi A, Zuccoli G, Nicolini A, Pipitone N, Macchioni L, Bajocchi GL, et 1T magnetic resonance imaging in the diagnosis of giant cell arteritis: comparison with ultrasonography and physical examination of temporal arteries. Clin Exp Rheumatol 2008;26 Suppl 49:S76–80.
- Hauenstein C, Reinhard M, Geiger J, Markl M, Hetzel A, Treszl A, et Effects of early corticosteroid treatment on magnetic resonance imaging and ultrasonography findings in giant cell arteritis. Rheumatology (Oxford) 2012;51:1999–2003.
- Klink T, Geiger J, Both M, Ness T, Heinzelmann S, Reinhard M, et Giant cell arteritis: diagnostic accuracy of MR imaging of superficial cranial arteries in initial diagnosis-results from a multi- center trial. Radiology 2014;273:844–52.
- Rhéaume M, Rebello R, Pagnoux C, Carette S, Clements-Baker M, Cohen-Hallaleh V, et High-resolution magnetic resonance imaging of scalp arteries for the diagnosis of giant cell arteritis: results of a prospective cohort study. Arthritis Rheumatol 2017;69:161–8.
- Blockmans D, de Ceuninck L, Vanderschueren S, Knockaert D, Mortelmans L, Bobbaers Repetitive 18F-fluorodeoxyglucose positron emission tomography in giant cell arteritis: a prospective study of 35 patients. Arthritis Rheum 2006;55:131–7.
- Hay B, Mariano-Goulart D, Bourdon A, Benkiran M, Vauchot F, de Verbizier D, et Diagnostic performance of 18F-FDG PET-CT for large vessel involvement assessment in patients with suspected giant cell arteritis and negative temporal artery biopsy. Ann Nucl Med 2019;33:512–20.
- Kermani TA, Diab S, Sreih AG, Cuthbertson D, Borchin R, Carette S, et Arterial lesions in giant cell arteritis: a longitudinal study. Semin Arthritis Rheum 2019;48:707–13.
- Nielsen BD, Gormsen LC, Hansen IT, Keller KK, Therkildsen P, Hauge Three days of high-dose glucocorticoid treatment attenuates large-vessel 18F-FDG uptake in large-vessel giant cell arteritis but with a limited impact on diagnostic accuracy. Eur J Nucl Med Mol Imaging 2018;45:1119–28.
- Pfadenhauer K, Weinerth J, Hrdina Vertebral arteries: a target for FDG-PET imaging in giant cell arteritis? Clinical, ultrasonographic and PET study in 46 patients. Nuklearmedizin 2011;50:28–32.
- Quinn KA, Ahlman MA, Malayeri AA, Marko J, Civelek AC, Rosenblum JS, et Comparison of magnetic resonance angiography and 18F-fluorodeoxyglucose positron emission tomography in large-vessel vasculitis. Ann Rheum Dis 2018;77:1165–71.
- Schmidt WA, Seifert A, Gromnica-Ihle E, Krause A, Natusch Ultrasound of proximal upper extremity arteries to increase the diagnostic yield in large-vessel giant cell arteritis. Rheumatology (Oxford) 2008;47:96–101.
- Grayson PC, Alehashemi S, Bagheri AA, Civelek AC, Cupps TR, Kaplan MJ, et 18F-fluorodeoxyglucose–positron emission tomography as an imaging biomarker in a prospective, longitudinal cohort of patients with large vessel vasculitis. Arthritis Rheumatol 2018;70:439–49.
- Grayson PC, Tomasson G, Cuthbertson D, Carette S, Hoffman GS, Khalidi NA, et Association of vascular physical examination findings and arteriographic lesions in large vessel vasculitis. J Rheumatol 2012;39:303–9.
- Chevalet P, Barrier JH, Pottier P, Hamidou M, Planchon B, El Kouri D, et A randomized, multicenter, controlled trial using intravenous pulses of methylprednisolone in the initial treatment of simple forms of giant cell arteritis: a one year followup study of 164 patients. J Rheumatol 2000;27:1484–91.
- Mazlumzadeh M, Hunder GG, Easley KA, Calamia KT, Matteson EL, Griffing WL, et Treatment of giant cell arteritis using induction therapy with high-dose glucocorticoids: a double-blind, placebo- controlled, randomized prospective clinical trial. Arthritis Rheum 2006;54:3310–8.
- Chan CC, Paine M, O’Day Steroid management in giant cell arteritis. Br J Ophthalmol 2001;85:1061–4.
- Hayreh SS, Zimmerman B, Kardon Visual improvement with corticosteroid therapy in giant cell arteritis: report of a large study and review of literature. Acta Ophthalmol Scand 2002;80:355–67.
- Hunder GG, Sheps SG, Allen GL, Joyce Daily and alternate- day corticosteroid regimens in treatment of giant cell arteritis: comparison in a prospective study. Ann Intern Med 1975;82:613–8.
- Delecoeuillerie G, Joly P, de Lara AC, Paolaggi Polymyalgia rheumatica and temporal arteritis: a retrospective analysis of prognostic features and different corticosteroid regimens (11 year sur- vey of 210 patients). Ann Rheum Dis 1988;47:733–9.
- Nesher G, Rubinow A, Sonnenblick Efficacy and adverse effects of different corticosteroid dose regimens in temporal arteritis: a retrospective study. Clin Exp Rheumatol 1997;15:303–6.
- Villiger PM, Adler S, Kuchen S, Wermelinger F, Dan D, Fiege V, et Tocilizumab for induction and maintenance of remission in giant cell arteritis: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet 2016;387:1921–7.
- Langford CA, Cuthbertson D, Ytterberg SR, Khalidi N, Monach PA, Carette S, et A randomized, double-blind trial of abatacept (CTLA-4Ig) for the treatment of giant cell arteritis. Arthritis Rheumatol 2017;69:837–45.
- Mahr AD, Jover JA, Spiera RF, Hernández-García C, Fernández- Gutiérrez B, LaValley MP, et Adjunctive methotrexate for treatment of giant cell arteritis: an individual patient data meta-analysis. Arthritis Rheum 2007;56:2789–97.
- Seror R, Baron G, Hachulla E, Debandt M, Larroche C, Puéchal X, et Adalimumab for steroid sparing in patients with giant-cell arteritis: results of a multicentre randomised controlled trial. Ann Rheum Dis 2014;73:2074–81.
- García-Martínez A, Hernández-Rodríguez J, Grau JM, Cid Treatment with statins does not exhibit a clinically relevant corticosteroid-sparing effect in patients with giant cell arteritis. Arthritis Rheum 2004;51:674–8.
- Narvaez J, Bernad B, Nolla JM, Valverde Statin therapy does not seem to benefit giant cell arteritis. Semin Arthritis Rheum 2007;36:322–7.
- Pugnet G, Sailler L, Fournier JP, Bourrel R, Montastruc JL, Lapeyre- Mestre Predictors of cardiovascular hospitalization in giant cell arteritis: effect of statin exposure. A French population-based study. J Rheumatol 2016;43:2162–70.
- Berger CT, Wolbers M, Meyer P, Daikeler T, Hess High incidence of severe ischaemic complications in patients with giant cell arteritis irrespective of platelet count and size, and platelet inhibition. Rheumatology (Oxford) 2009;48:258–61.
- Narvaez J, Bernad B, Gómez-Vaquero C, García-Gómez C, Roig- Vilaseca D, Juanola X, et Impact of antiplatelet therapy in the development of severe ischemic complications and in the outcome of patients with giant cell arteritis. Clin Exp Rheumatol 2008;26 Suppl 49:S57–62.
- Nesher G, Berkun Y, Mates M, Baras M, Rubinow A, Sonnenblick Low-dose aspirin and prevention of cranial ischemic complications in giant cell arteritis. Arthritis Rheum 2004;50:1332–7.
- Salvarani C, Della Bella C, Cimino L, Macchioni P, Formisano D, Bajocchi G, et Risk factors for severe cranial ischaemic events in an Italian population-based cohort of patients with giant cell arteritis. Rheumatology (Oxford) 2009;48:250–3.
- Hoffman GS, Cid MC, Hellmann DB, Guillevin L, Stone JH, Schousboe J, et A multicenter, randomized, double-blind, placebo-controlled trial of adjuvant methotrexate treatment for giant cell arteritis. Arthritis Rheum 2002;46:1309–18.
- Spiera RF, Mitnick HJ, Kupersmith M, Richmond M, Spiera H, Peterson MG, et A prospective, double-blind, randomized, placebo controlled trial of methotrexate in the treatment of giant cell arteritis (GCA). Clin Exp Rheumatol 2001;19:495–501.
- Hoffman GS, Cid MC, Rendt-Zagar KE, Merkel PA, Weyand CM, Stone JH, et Infliximab for maintenance of glucocorticosteroid-induced remission of giant cell arteritis: a randomized trial. Ann Intern Med 2007;146:621–30.
- Both M, Aries PM, Muller-Hulsbeck S, Jahnke T, Schäfer PJ, Gross WL, et Balloon angioplasty of arteries of the upper extremities in patients with extracranial giant-cell arteritis. Ann Rheum Dis 2006;65:1124–30.
- Clifford AH, Arafat A, Idrees JJ, Roselli EE, Tan CD, Rodriguez R, et Outcomes among 196 patients with noninfectious proximal aortitis. Arthritis Rheumatol 2019;71:2112–20.
- Mennander AA, Miller DV, Liang KP, Warrington KJ, Connolly HM, Schaff HV, et Surgical management of ascending aortic aneurysm due to non-infectious aortitis. Scand Cardiovasc J 2008;42:417–24.
- Allen DB, Growth suppression by glucocorticoid therapy [review]. Endocrinol Metab Clin North Am 1996;25:699–717.
- Mutoh T, Shirai T, Fujii H, Ishii T, Harigae Insufficient use of corticosteroids without immunosuppressants results in higher relapse rates in Takayasu arteritis. J Rheumatol 2020;47:255–63.
- Molloy ES, Langford CA, Clark TM, Gota CE, Hoffman Anti-tumour necrosis factor therapy in patients with refractory Takayasu arteritis: long-term follow-up. Ann Rheum Dis 2008;67:1567–9.
- Nakaoka Y, Isobe M, Takei S, Tanaka Y, Ishii T, Yokota S, et Efficacy and safety of tocilizumab in patients with refractory Takayasu arteritis: results from a randomised, double-blind, placebo-controlled, phase 3 trial in Japan (the TAKT study). Ann Rheum Dis 2018;77:348–54.
- Mekinian A, Resche-Rigon M, Comarmond C, Soriano A, Constans J, Alric L, et Efficacy of tocilizumab in Takayasu arteritis: multicenter retrospective study of 46 patients. J Autoimmun 2018;91:55–60.
- Langford CA, Cuthbertson D, Ytterberg SR, Khalidi N, Monach PA, Carette S, et A randomized, double-blind trial of abatacept (CTLA-4Ig) for the treatment of Takayasu arteritis. Arthritis Rheumatol 2017;69:846–53.
- Aeschlimann FA, Eng SW, Sheikh S, Laxer RM, Hebert D, Noone D, et Childhood Takayasu arteritis: disease course and response to therapy. Arthritis Res Ther 2017;19:255.
- Mekinian A, Comarmond C, Resche-Rigon M, Mirault T, Kahn JE, Lambert M, et Efficacy of biological-targeted treatments in Takayasu arteritis: multicenter, retrospective study of 49 patients. Circulation 2015;132:1693–700.
- Schmidt J, Kermani TA, Bacani AK, Crowson CS, Matteson EL, Warrington Tumor necrosis factor inhibitors in patients with Takayasu arteritis: experience from a referral center with long-term followup. Arthritis Care Res (Hoboken) 2012;64:1079–83.
- Comarmond C, Biard L, Lambert M, Mekinian A, Ferfar Y, Kahn JE, et Long-term outcomes and prognostic factors of complications in Takayasu arteritis: a multicenter study of 318 patients. Circulation 2017;136:1114–22.
- Gulcu A, Gezer NS, Akar S, Akkoc N, Onen F, Goktay AY. Long-term follow-up of endovascular repair in the management of arterial stenosis caused by Takayasu’s Ann Vasc Surg 2017;42:93–100.
- De Souza AW, Machado NP, Pereira VM, Arraes AE, Neto ET, Maria HA, et Antiplatelet therapy for the prevention of arterial ischemic events in Takayasu arteritis. Circ J 2010;74:1236–41.
- Wang X, Dang A, Lv N, Liu Q, Chen High-sensitivity C-reactive protein predicts adverse cardiovascular events in patients with Takayasu arteritis with coronary artery involvement. Clin Rheumatol 2016;35:679–84.
- Liu YQ, Ling J, Wang Intravenous digital subtraction angiography in patients with aorto-arteritis (Takayasu’s). Cardiovasc Intervent Radiol 1990;13:83–7.
- Lee KH, Cho A, Choi YJ, Lee SW, Ha YJ, Jung SJ, et The role of 18F-fluorodeoxyglucose–positron emission tomography in the assessment of disease activity in patients with Takayasu arteritis. Arthritis Rheum 2012;64:866–75.
- Walter MA, Melzer RA, Schindler C, Muller-Brand J, Tyndall A, Nitzsche The value of [18F]FDG-PET in the diagnosis of large- vessel vasculitis and the assessment of activity and extent of disease. Eur J Nucl Med Mol Imaging 2005;32:674–81.
- Vinicki JP, Garcia-Vicuna R, Arredondo M, López-Bote JP, García- Vadillo JA, Castaneda S, et Sustained remission after long-term biological therapy in patients with large vessel vasculitis: an analysis of ten cases. Reumatol Clin 2017;13:210–3.
- Ando M, Sasako Y, Okita Y, Tagusari O, Kitamura S, Matsuo Surgical considerations of occlusive lesions associated with Takayasu’s arteritis. Jpn J Thorac Cardiovasc Surg 2000;48:173–9.
- Lee GY, Jeon P, Do YS, Sung K, Kim DI, Kim YW, et Comparison of outcomes between endovascular treatment and bypass surgery in Takayasu arteritis. Scand J Rheumatol 2014;43:153–61.
- Zheng T, Zhu S, Ou JF, Fang WG, Qiao ZY, Qi RD, et Treatment with corticosteroid and/or immunosuppressive agents before surgery can effectively improve the surgical outcome in patients with Takayasu’s arteritis. J Invest Surg2019;32:220–7.
- Ham SW, Weaver Ex vivo renal artery reconstruction for complex renal artery disease. J Vasc Surg 2014;60:143–50.
- Khalilullah M, Tyagi Percutaneous transluminal angioplasty in Takayasu arteritis. Heart Vessels Suppl 1992;7:146–53.
- Sharma S, Gupta H, Saxena A, Kothari SS, Taneja K, Guleria S, et Results of renal angioplasty in nonspecific aortoarteritis (Takayasu disease). J Vasc Interv Radiol 1998;9:429–35.
- Sun Y, Ma L, Ma L, Kong X, Chen H, Lv P, et Cyclophosphamide could be a better choice than methotrexate as induction treatment for patients with more severe Takayasu’s arteritis. Rheumatol Int 2017;37:2019–26.
- Chen B, Yu HX, Zhang J, Li XX, Wu XG, Yang SJ, et Endovascular revascularization for carotid artery occlusion in patients with Takayasu arteritis. Eur J Vasc Endovasc Surg 2015;49:498–505.
- Fields CE, Bower TC, Cooper LT, Hoskin T, Noel AA, Panneton JM, et Takayasu’s arteritis: operative results and influence of disease activity. J Vasc Surg 2006;43:64–71.
- Kim HJ, Lee CS, Kim JS, Know SU, Kim JL, Park JW, et Outcomes after endovascular treatment of symptomatic patients with Takayasu’s arteritis. Interv Neuroradiol 2011;17:252–60.
- Kim YW, Kim DI, Park YJ, Yang SS, Lee GY, Kim DK, et Surgical bypass vs endovascular treatment for patients with supra-aortic arterial occlusive disease due to Takayasu arteritis. J Vasc Surg 2012;55:693–700.
- Kinjo H, Kafa The results of treatment in renal artery stenosis due to Takayasu disease: comparison between surgery, angioplasty, and stenting. A monocentrique retrospective study. G Chir 2015;36:161–7.
- Park MC, Lee SW, Park YB, Lee SK, Choi D, Shim Post- interventional immunosuppressive treatment and vascular restenosis in Takayasu’s arteritis. Rheumatology (Oxford) 2006;45:600–5.
- Wang X, Dang A, Lv N, Cheng N, Cheng X, Yang Y, et Long- term outcomes of coronary artery bypass grafting versus per- cutaneous coronary intervention for Takayasu arteritis patients with coronary artery involvement. Semin Arthritis Rheum 2017;47:247–52.
- Ham SW, Kumar SR, Wang BR, Rowe VL, Weaver Late out- comes of endovascular and open revascularization for nonatherosclerotic renal artery disease. Arch Surg 2010;145:832–9.
- Dejaco C, Ramiro S, Duftner C, Besson FL, Bley TA, Blockmans D, et EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice. Ann Rheum Dis 2018;77:636–43.
- Best JH, Kong AM, Unizony S, Tran O, Michalska Risk of potential glucocorticoid-related adverse events in patients with giant cell arteritis: results from a USA-based electronic health records data- base. Rheumatol Ther 2019;6:599–610.
- Hellmich B, Agueda A, Monti S, Buttgereit F, de Boysson H, Brouwer E, et 2018 Update of the EULAR recommendations for the management of large vessel vasculitis. Ann Rheum Dis 2020;79:19–30.
Results
We present 22 recommendations and 2 ungraded position statements for GCA, and 20 recommendations and 1 ungraded position statement for TAK. These recommendations and statements address clinical questions relating to the use of diagnostic testing, including imaging, treatments, and surgical interventions in GCA and TAK. Recommendations for GCA include support for the use of glucocorticoid-sparing immunosuppressive agents and the use of imaging to identify large vessel involvement. Recommendations for TAK include the use of nonglucocorticoid immunosuppressive agents with glucocorticoids as initial therapy. There were only 2 strong recommendations; the remaining recommendations were conditional due to the low quality of evidence available for most PICO questions.
Recommendations and Ungraded Positions for GCA
Table 1 presents definitions of selected terms used in the recommendations, including disease states such as severe disease, dosing ranges for glucocorticoids, categorization of medications, and disease assessments. Tables 2 and 3 present the recommendations with their supporting PICO questions and levels of evidence. We present 22 recommendations and 2 ungraded position statements for GCA. All but 1 of the recommendations are conditional due to very low– to low-quality evidence. Figure 1 presents key recommendations for the treatment of GCA.
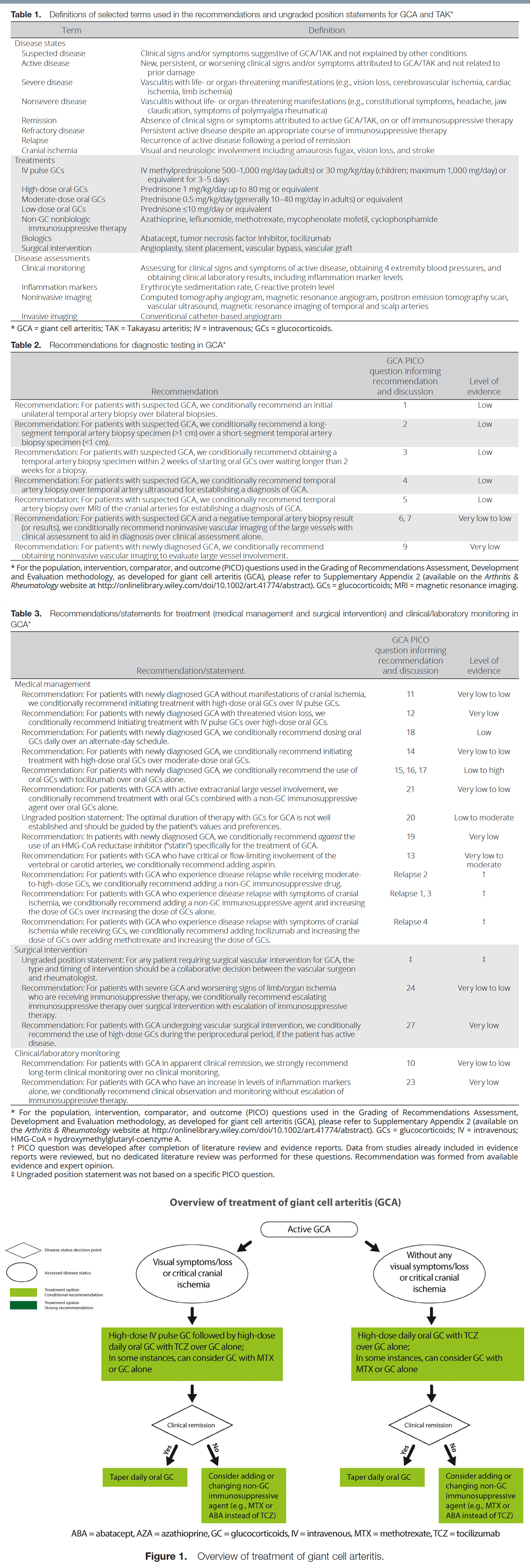
Recommendation: For patients with suspected GCA, we conditionally recommend an initial unilateral temporal artery biopsy over bilateral biopsies.
Initially, a unilateral biopsy is recommended. However, bilateral temporal artery biopsies may be appropriate if the symptoms are not clearly localized to 1 temporal artery. Proceeding with the contralateral biopsy is also appropriate if the unilateral biopsy result is negative and additional evidence for cranial GCA is sought (17).
Recommendation: For patients with suspected GCA, we conditionally recommend a long-segment temporal artery biopsy specimen (>1 cm) over a short-segment temporal artery biopsy specimen (<1 cm).
A longer segment of the temporal artery is preferred, since GCA is a focal and segmental disease, and the added morbidity of obtaining a larger segment is very low. A shorter segment obtained on biopsy can result in reduced diagnostic yield and a missed diagnosis. This recommendation is conditional due to a lack of high-quality evidence, but the Voting Panel emphasized obtaining longer biopsy specimens when possible (18,19).
Recommendation: For patients with suspected GCA, we conditionally recommend obtaining a temporal artery biopsy specimen within 2 weeks of starting oral glucocorticoids over waiting longer than 2 weeks for a biopsy.
Overall, biopsy specimens should be obtained as soon as possible to maximize the likelihood of detecting histopathologic changes. Studies suggest that histopathologic changes indicating GCA are more likely to be detected in a temporal artery biopsy if obtained within 2 weeks of starting glucocorticoids; however, histopathologic changes have been detected in biopsy specimens obtained much later than 2 weeks after the start of glucocorticoid treatment (20–28). A biopsy specimen obtained 2 weeks after starting glucocorticoids could be informative and may be considered at the discretion of the physician and patient.
Recommendation: For patients with suspected GCA, we conditionally recommend temporal artery biopsy over temporal artery ultrasound for establishing a diagnosis of GCA.
In general, rheumatologists and radiologists in the US are less experienced in using ultrasound to diagnose temporal artery involvement in GCA compared to their counterparts in Europe. Therefore, temporal artery biopsy remains the optimal approach to diagnosing GCA in the US, because ultrasound is operator- dependent and results are influenced by treatment (i.e., signs of inflammation quickly disappear with glucocorticoid treatment). In centers with appropriate training and expertise in using temporal artery ultrasound, ultrasound may be a useful and complementary tool for diagnosing GCA (29–33).
Recommendation: For patients with suspected GCA, we conditionally recommend temporal artery biopsy over magnetic resonance imaging (MRI) of the cranial arteries for establishing a diagnosis of GCA.
Protocols to image the cranial vessels using different modalities, including MRI, have been developed, which can be helpful to establish a diagnosis of GCA (30,31,34– 37). However, lack of technical expertise with this modality in the US, as well as the lack of widespread validation of this approach, limits the applicability of MRI with contrast of the cranial vessels as a replacement for temporal artery biopsy at the current time.
Recommendation: For patients with suspected GCA and a negative temporal artery biopsy result (or results), we conditionally recommend noninvasive vascular imaging of the large vessels with clinical assessment to aid in diagnosis over clinical assessment alone.
Imaging the large vessels may provide additional evidence of disease (e.g., extracranial GCA) when the diagnosis is uncertain following negative temporal artery biopsy results (28,34,38–44). Potential diagnostic imaging modalities include MR or computed tomography (CT) angiography of the neck/chest/abdomen/pelvis, ultrasonography, and 18F-fluorodeoxyglucose positron emission tomography (FDG- PET) (43,45).
Recommendation: For patients with newly diagnosed GCA, we conditionally recommend obtaining noninvasive vascular imaging to evaluate large vessel involvement.
Baseline noninvasive imaging with MR or CT angiography of the neck/chest/abdomen/pelvis in patients with newly diagnosed GCA can detect large vessel involvement and may be compared with subsequent routine monitoring if indicated (46). In a patient with large vessel involvement, routine noninvasive vascular imaging can identify early and long-term complications, such as aneurysms and stenoses, and assess stability of existing lesions. In patients without large vessel involvement, routine and repeated monitoring with vascular imaging may or may not be necessary.
Recommendation: For patients with newly diagnosed GCA without manifestations of cranial ischemia, we conditionally recommend initiating treatment with high-dose oral glucocorticoids over intravenous (IV) pulse glucocorticoids.
Cranial ischemic manifestations include visual and neurologic involvement such as amaurosis fugax, vision loss, and stroke. Some studies have suggested that the use of IV pulse glucocorticoids in this patient group could decrease disease relapse and increase remission rates. However, routine use of IV pulse glucocorticoids can also be associated with increased risks, including infections, that may outweigh the benefits, especially in the elderly (47,48).
Recommendation: For patients with newly diagnosed GCA with threatened vision loss, we conditionally recommend initiating treatment with IV pulse glucocorticoids over high-dose oral glucocorticoids.
Studies investigating the effect of IV pulse glucocorticoids in patients with GCA and cranial ischemia have demonstrated conflicting results. However, this population is at high risk for vision loss as well as toxicity from glucocorticoid use. IV pulse glucocorticoids can be used in patients with the highest risk of vision loss, but this decision should be guided by the patient’s clinical condition, values, and preferences (49,50).
Recommendation: For patients with newly diagnosed GCA, we conditionally recommend dosing oral glucocorticoids daily over an alternate-day schedule.
This recommendation is conditional solely due to the low level of evidence, which indicates higher remission rates in patients receiving daily dosing. The panel did not identify any situations in which alternate-day dosing of prednisone would be preferred (51).
Recommendation: For patients with newly diagnosed GCA, we conditionally recommend initiating treatment with high-dose oral glucocorticoids over moderate-dose oral glucocorticoids.
We recommend starting high-dose oral glucocorticoids to achieve rapid disease control followed by tapering the glucocorticoid dose (weeks to months) to avoid prolonged high- dose treatment and reduce toxicity. The dosing and duration of oral glucocorticoid therapy can be variable depending on a patient’s manifestations and comorbidities and whether the use of a glucocorticoid-sparing agent was also initiated. Studies supporting the efficacy and lower toxicity of moderate-dose glucocorticoids are of low quality, which prevents the Voting Panel from recommending moderate-dose glucocorticoids as initial therapy. Moderate-dose glucocorticoids may be used in patients with significant risk of severe glucocorticoid toxicity and in patients with low risk of vision loss or other life- or organ-threatening complications (48–53).
Recommendation: For patients with newly diagnosed GCA, we conditionally recommend the use of oral glucocorticoids with tocilizumab over oral glucocorticoids alone.
A trial published in 2017 (8) demonstrated that tocilizumab has a significant glucocorticoid-sparing effect in GCA, and thus, tocilizumab should be considered for initial treatment. However, methotrexate with glucocorticoids, as well as glucocorticoids alone, can also be considered as initial treatment for newly diagnosed GCA. The decision to treat with tocilizumab and glucocorticoids, methotrexate and glucocorticoids, or glucocorticoid monotherapy for initial therapy should be made based on the physician’s experience and the patient’s clinical condition, values, and preferences. Lack of long-term follow-up data on tocilizumab and cost may limit its use (8,54). Abatacept with glucocorticoids can also be considered if these other agents are not effective (55).
Recommendation: For patients with GCA with active extracranial large vessel involvement, we conditionally recommend treatment with oral glucocorticoids combined with a nonglucocorticoid immunosuppressive agent over oral glucocorticoids alone.
Management of GCA in patients with new, persistent, or worsening extracranial symptoms (e.g., limb claudication) or signs (e.g., imaging findings) attributed to GCA can include the addition of nonglucocorticoid immunosuppressive agents. These agents include biologic agents (e.g., tocilizumab) as well as oral therapies (e.g., methotrexate) (56,57). However, the Voting Panel recognizes that there are few high-quality studies evaluating the efficacy of these agents for this patient group. While there is stronger clinical evidence supporting the use of tocilizumab compared to methotrexate for the treatment of GCA, methotrexate can be considered for patients unable to use tocilizumab due to factors such as recurrent infections, history of gastrointestinal perforations or diverticulitis, and cost.
Ungraded position statement: The optimal duration of therapy with glucocorticoids for GCA is not well established and should be guided by the patient’s values and preferences.
Factors that may influence the duration of therapy include the patient’s clinical manifestations, toxicity related to glucocorticoid use, number of flares, the physician’s experience, and the patient’s preferences (8). Overall, the Patient Panel emphasized minimizing the use of glucocorticoids as much as possible but recognized that longer-term use may be needed in some patients to avoid flares.
Recommendation: In patients with newly diagnosed GCA, we conditionally recommend against the use of a hydroxymethylglutaryl-coenzyme A reductase inhibitor (“statin”) specifically for the treatment of GCA.
The use of statins is not known to provide a clinically significant immunosuppressive effect for GCA. Whether statins are warranted to decrease the patient’s risk of cardiovascular events is a separate clinical question and depends on the patient’s risk factors for cardiovascular disease (58–60).
Recommendation: For patients with GCA who have critical or flow-limiting involvement of the vertebral or carotid arteries, we conditionally recommend adding aspirin.
There are few data regarding this clinical question, but the antiplatelet activity of aspirin may be beneficial in preventing ischemic events in patients with vascular narrowing causing decreased cerebral blood flow (61–64). The efficacy of aspirin to prevent ischemic events in patients without vertebral or carotid narrowing remains unclear at this time.
Recommendation: For patients with GCA who experience disease relapse while receiving moderate-to-high– dose glucocorticoids, we conditionally recommend adding a nonglucocorticoid immunosuppressive drug.
Relapses of any type while receiving moderate-to-high–dose glucocorticoids indicate that it is unlikely that it will be possible for glucocorticoids to be tapered to a low dose. Therefore, glucocorticoid-sparing therapy should be considered.
Recommendation: For patients with GCA who experience disease relapse with symptoms of cranial ischemia, we conditionally recommend adding a nonglucocorticoid immunosuppressive agent and increasing the dose of glucocorticoids over increasing the dose of glucocorticoids alone.
Nonglucocorticoid immunosuppressive agents considered in this situation include tocilizumab and methotrexate (8,65,66). Relapses with symptoms of polymyalgia rheumatica may be controlled by increasing the dose of glucocorticoids alone.
Recommendation: For patients with GCA who experience disease relapse with cranial symptoms while receiving glucocorticoids, we conditionally recommend adding tocilizumab and increasing the dose of glucocorticoids over adding methotrexate and increasing the dose of glucocorticoids.
Tocilizumab is an effective glucocorticoid-sparing agent for GCA (8,54). While there are no comparative studies, the glucocorticoid- sparing effect seen with methotrexate is smaller than the effect seen with tocilizumab (8,55,65–67). While the glucocorticoid-sparing effect of tocilizumab is best quantified using the subcutaneous formulation (8), IV tocilizumab has also been shown to be glucocorticoid-sparing (54). Again, methotrexate can be considered for patients who are unable to tolerate or have limited access to tocilizumab.
Ungraded position statement: For any patient requiring surgical vascular intervention for GCA, the type and timing of intervention should be a collaborative decision between the vascular surgeon and rheumatologist.
Recommendation: For patients with severe GCA and worsening signs of limb/organ ischemia who are receiving immunosuppressive therapy, we conditionally recommend escalating immunosuppressive therapy over surgical intervention with escalation of immunosuppressive therapy.
Because patients can develop collateral blood vessels to improve distal blood flow, immunosuppressive therapy is recommended as initial therapy in patients with GCA and worsening limb/organ ischemia. However, clinical situations that would warrant consideration of immediate surgical intervention include aortic aneurysms at high risk for rupture and impending/progressive tissue or organ infarction or damage (68–70).
Recommendation: For patients with GCA undergoing vascular surgical intervention, we conditionally recommend the use of high-dose glucocorticoids during the periprocedural period, if the patient has active disease.
This recommendation pertains to patients with GCA who are undergoing a vascular surgical intervention due to a complication of GCA (e.g., aneurysm or stenosis). There are limited data regarding the use of high-dose glucocorticoids during the periprocedural period in GCA, and thus, support for this recommendation is based in part on their use in TAK. As in TAK, high doses of oral glucocorticoids in the perioperative setting are recommended if the disease is active or if the clinician is concerned that the patient may have active disease.
Clinical/laboratory Monitoring for GCA
Recommendation: For patients with GCA in apparent clinical remission, we strongly recommend long-term clinical monitoring over no clinical monitoring.
The optimal frequency and length of monitoring are not well established and depend on factors including the duration of remission, site of involvement, risk of disease progression, whether the patient is receiving immunosuppressive therapy, and reliability of the patient to report new signs or symptoms (48,69). Clinical monitoring may include history taking, examinations, and laboratory and imaging studies. This is a strong recommendation given the minimal risks and potential catastrophic outcomes if monitoring is not performed.
Recommendation: For patients with GCA who have an increase in levels of inflammation markers alone, we conditionally recommend clinical observation and monitoring without escalation of immunosuppressive therapy.
Increases in levels of inflammation markers such as erythrocyte sedimentation rate and C-reactive protein can be non-specific (69). Therefore, increasing immunosuppressive therapy is not warranted in the setting of increased levels of inflammation markers in the absence of other signs of disease activity. However, these increased levels may warrant more frequent clinical and/or radiographic assessments for active disease.
Recommendations and Ungraded Positions for TAK
Table 1 presents definitions of selected terms used in the recommendations, and Tables 4 and 5 present the recommendations with their supporting PICO questions and levels of evidence. We present 20 recommendations and 1 ungraded position statement for TAK. All recommendations except for 1 are conditional due to the availability of only very low– to low-quality evidence. Figure 2 presents key recommendations for the treatment of TAK.
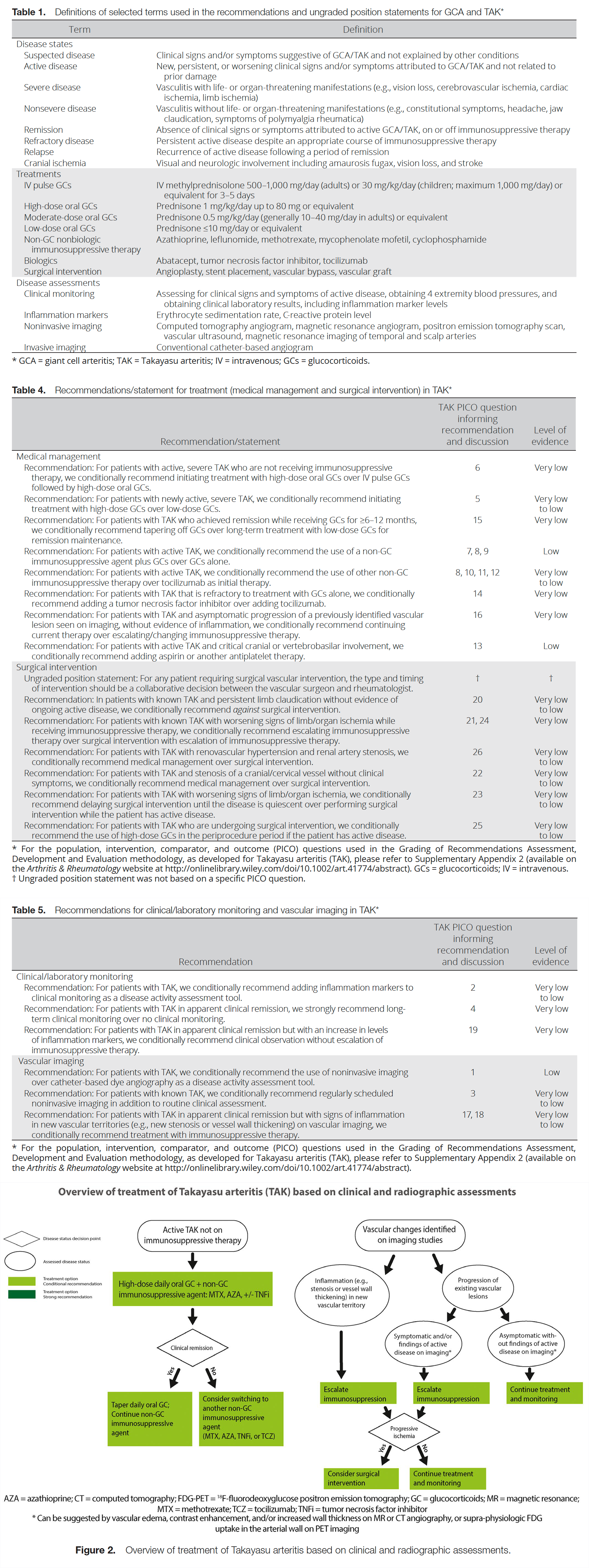
Recommendation: For patients with active, severe TAK who are not receiving immunosuppressive therapy, we conditionally recommend initiating treatment with high-dose oral glucocorticoids over IV pulse glucocorticoids followed by high-dose oral glucocorticoids.
There is no evidence that IV pulse glucocorticoids are more effective than high-dose oral glucocorticoids in this setting. IV pulse glucocorticoids may be considered for patients with life- or organ-threatening disease. In children, alternate steroid dosing regimens (e.g., IV pulse glucocorticoids with low daily oral dosing) may be preferred to improve compliance and potentially reduce adverse consequences such as impacting growth (71).
Recommendation: For patients with newly active, severe TAK, we conditionally recommend initiating treatment with high-dose glucocorticoids over low-dose glucocorticoids.
A higher dose of glucocorticoids is recommended due to the potential for organ damage or life-threatening events. However, lower doses of glucocorticoids may be considered for patients with newly active, nonsevere disease (e.g., patients with constitutional symptoms and without limb ischemia) (72).
Recommendation: For patients with TAK who achieved remission while receiving glucocorticoids for ≥6–12 months, we conditionally recommend tapering off glucocorticoids over long-term treatment with low-dose glucocorticoids for remission maintenance.
The optimal duration of glucocorticoid use in TAK is unknown. Glucocorticoid exposure should be limited if possible in order to minimize toxicity. Glucocorticoids may be continued for a longer duration if disease is not adequately controlled or if the patient experiences frequent disease relapse.
Recommendation: For patients with active TAK, we conditionally recommend the use of a nonglucocorticoid immunosuppressive agent plus glucocorticoids over glucocorticoids alone.
Nonglucocorticoid immunosuppressive agents are recommended over monotherapy with glucocorticoids to minimize glucocorticoid-related toxicity. Methotrexate is often used as the initial nonglucocorticoid immunosuppressive agent, but other therapies such as tumor necrosis factor inhibitors and azathioprine can be considered as well (70–73). Methotrexate is often preferred for use in children since it is usually well tolerated. Glucocorticoid monotherapy can be considered for mild disease or if the diagnosis is uncertain. Patient-specific factors such as alcohol use, plans for childbearing, medication compliance, and medical comorbidities may influence the choice of immunosuppressant (73,74).
Recommendation: For patients with active TAK, we conditionally recommend the use of other nonglucocorticoid immunosuppressive therapy over tocilizumab as initial therapy.
As discussed above, nonglucocorticoid immunosuppressive agents such as methotrexate, tumor necrosis factor inhibitors, and azathioprine can be used as initial therapy in TAK. We recommend these agents over tocilizumab for initial therapy, because the efficacy of tocilizumab in TAK is not established at this time. While tocilizumab has been shown to be efficacious for GCA, the primary efficacy end point was not achieved in the only randomized trial of tocilizumab in TAK conducted thus far (74,75). Tocilizumab may be considered for patients with inadequate response to other immunosuppressive therapies. Abatacept is not recommended, since it has been shown in a small randomized controlled trial to not be efficacious in TAK (74,76).
Recommendation: For patients with TAK that is refractory to treatment with glucocorticoids alone, we conditionally recommend adding a tumor necrosis factor inhibitor over adding tocilizumab.
We recognize that among biologic therapies, some practitioners favor TNF inhibition, while others favor interleukin-6 inhibition (tocilizumab) in this situation. Overall, the Voting Panel favored tumor necrosis factor inhibitors over tocilizumab, since there is more clinical experience with and data on tumor necrosis factor inhibitors in TAK compared to tocilizumab. In observational studies, tumor necrosis factor inhibitors have been shown to induce remission and decrease relapses (77–79). Clinical experience with tocilizumab in TAK has been demonstrated in a randomized controlled trial and small case series. In the randomized trial, a trend toward a longer time to relapse was seen in the tocilizumab arm, but the difference was not statistically significant. However, that study was felt to be underpowered (36 participants). Of note, tocilizumab use also affects acute-phase reactants, which may impact ability to gauge disease activity. Therefore, while the panel favors tumor necrosis factor inhibitor use, we recognize that tocilizumab may also be considered, especially when tumor necrosis factor inhibitors are contraindicated (75).
Recommendation: For patients with TAK and asymptomatic progression of a previously identified vascular lesion seen on imaging, without evidence of inflammation, we conditionally recommend continuing current therapy over escalating/changing immunosuppressive therapy.
Vascular lesions can progress due to a number of factors that may not be related to active disease, such as “healing fibrosis” in response to effective treatment. Intervention is not always needed, since collateral circulation frequently develops over time. However, the location and the extent of the lesion of the affected vessel should be considered. Escalating immunosuppressive therapy may be warranted if significant progression has developed rapidly (e.g., weeks to months) after a period of stable disease (80,81).
Recommendation: For patients with active TAK and critical cranial or vertebrobasilar involvement, we conditionally recommend adding aspirin or another antiplatelet therapy.
Small observational studies suggest a decreased risk of ischemic events with antiplatelet therapy but an increased risk of bleeding (82). Therefore, antiplatelet therapy is usually used for patients at higher risk of ischemic events (e.g., patients with flow-limiting vertebrobasilar disease or stents). Antiplatelet therapy should be used with caution after surgical procedures or if there is an increased risk of bleeding (81).
Clinical/laboratory Monitoring for TAK
Recommendation: For patients with TAK, we conditionally recommend adding inflammation markers to clinical monitoring as a disease activity assessment tool.
While inflammation markers are an imperfect indicator of disease activity, they may be helpful for clinical monitoring (80,83).
Recommendation: For patients with TAK in apparent clinical remission, we strongly recommend long-term clinical monitoring over no clinical monitoring.
The frequency of monitoring depends on factors including the duration of remission, sites of involvement, risk of disease progression, the patient’s immunosuppressive regimen, and the ability and likelihood of the patient reliably reporting new signs or symptoms of TAK. This is a strong recommendation given the minimal risks and potential catastrophic outcomes without monitoring (80,83).
Recommendation: For patients with TAK in apparent clinical remission but with an increase in levels of inflammation markers, we conditionally recommend clinical observation without escalation of immunosuppressive therapy.
As discussed above in the GCA recommendations, increases in levels of inflammation markers can be nonspecific, and intensifying immunosuppressive therapy in the setting of increased inflammation markers alone may not be warranted. More frequent clinical and/or radiographic assessments for active disease can be considered (77,80,83).
Recommendation: For patients with TAK, we conditionally recommend the use of noninvasive imaging over catheter-based dye angiography as a disease activity assessment tool.
Noninvasive imaging such as CT angiography, MR angiography, or FDG-PET are recommended because these imaging modalities provide information regarding vascular wall inflammation, while catheter-based angiography primarily provides information regarding the vascular lumen. Catheter-based angiography can be used to accurately determine central blood pressures, as part of surgical planning, or if noninvasive modalities do not provide adequate information. Identifying active disease based on noninvasive imaging at this time can be challenging, since the hallmarks of active disease have not been definitively established (43,45,84).
Recommendation: For patients with known TAK, we conditionally recommend regularly scheduled noninvasive imaging in addition to routine clinical assessment.
Routine imaging is recommended since vascular changes in TAK can occur when the disease is considered clinically quiescent. The optimal interval between imaging studies is not well established, and ranges vary (e.g., every 3–6 months or longer). The interval may be shorter early in the disease course and longer with established, quiescent disease. Since sedation may be required for imaging studies in children and can be associated with potential risks and complications, routine imaging of inactive disease in children is at the discretion of the treating clinician, while considering risks and benefits (85,86).
Recommendation: For patients with TAK in apparent clinical remission but with signs of inflammation in new vascular territories (e.g., new stenosis or vessel wall thickening) on vascular imaging, we conditionally recommend treatment with immunosuppressive therapy.
A new arterial stenosis is concerning as it can indicate recent active disease, and thus usually warrants immunosuppressive therapy. Other findings suggestive of active disease on MR angiography or CT angiography include vascular edema, contrast enhancement, and increased wall thickness, and may result in luminal damage over time. Findings of active disease by FDG-PET are defined by supraphysiologic FDG uptake in the arterial wall. However, abnormal findings in the vascular wall identified by imaging are not necessarily specific to vascular inflammation. The implication of finding vessel wall edema or enhancement on imaging remains an area of investigation, and the clinical importance of such findings on CT angiography, MR angiography, or FDG-PET is not certain (43,45,80,83–86). Therefore, all therapeutic decision-making in this context should occur after reviewing the imaging findings with a radiologist to help determine whether the observed imaging changes represent active disease.
Ungraded position statement: For any patient requiring surgical vascular intervention, the type and timing of intervention should be a collaborative decision between the vascular surgeon and rheumatologist.
Recommendation: In patients with known TAK and persistent limb claudication without evidence of ongoing active disease, we conditionally recommend against surgical intervention.
Patients with TAK can develop collateral circulation that bypasses the stenosis causing limb claudication, and thus, surgical intervention may not be needed (87). However, surgical intervention can be considered for patients whose activities are significantly impacted by limb claudication.
Recommendation: For patients with known TAK with worsening signs of limb/organ ischemia while receiving immunosuppressive therapy, we conditionally recommend escalating immunosuppressive therapy over surgical intervention with escalation of immunosuppressive therapy.
Immunosuppressive therapy is recommended to control vascular inflammation in order to improve or prevent worsening blood flow. However, clinical situations that could warrant immediate surgical intervention include coronary artery involvement and impending/progressive tissue or organ infarction (88–90).
Recommendation: For patients with TAK with renovascular hypertension and renal artery stenosis, we conditionally recommend medical management over surgical intervention.
Medical management includes antihypertensive drugs and immunosuppressive therapy if TAK is active. Surgical intervention (including catheter-based interventions) may be warranted for hypertension that is refractory to medical management in spite of optimized immunosuppressive therapy or in the setting of worsening renal function (12,91–94).
Recommendation: For patients with TAK and stenosis of a cranial/cervical vessel without clinical symptoms, we conditionally recommend medical management over surgical intervention.
Medical therapy is recommended if only a single vessel is involved, due to the substantial risks of surgery. Surgical interventions can be considered if multiple vessels are involved. This recommendation is based on indirect evidence obtained from neurologic experience and studies, because there is no direct evidence for TAK (90,95–98).
Recommendation: For patients with TAK with worsening signs of limb/organ ischemia, we conditionally recommend delaying surgical intervention until the disease is quiescent over performing surgical intervention while the patient has active disease.
Observational studies have suggested improved outcomes if surgical intervention is performed when disease is not active. However, surgical intervention during active disease may be necessary if the patient has life- or organ-threatening manifestations such as stroke, loss of viability of a limb, or myocardial ischemia (99–101). We recognize that determining the level of disease activity in TAK can be challenging.
Recommendation: For patients with TAK who are undergoing surgical intervention, we conditionally recommend the use of high-dose glucocorticoids in the periprocedure period if the patient has active disease.
This recommendation pertains to patients with TAK who are undergoing a vascular surgical intervention due to a complication of TAK. High doses of oral glucocorticoids in the perioperative setting are recommended if the disease is active or if the clinician is concerned that the patient may have active disease (90,96,102).
